Glecirasib (JAB-21822) is a potential best-in-class KRAS G12C allosteric inhibitor independently developed by Jacobio with promising early clinical efficacy. Glecirasib can be used as monotherapy to treat non-small cell lung cancer, colorectal cancer and other solid tumors with the KRAS G12C mutation. Combinations of Glecirasib with SHP2i, EGFRi, or PD-1 antibodies is expected to have synergistic efficacy and overcome acquired drug resistance.
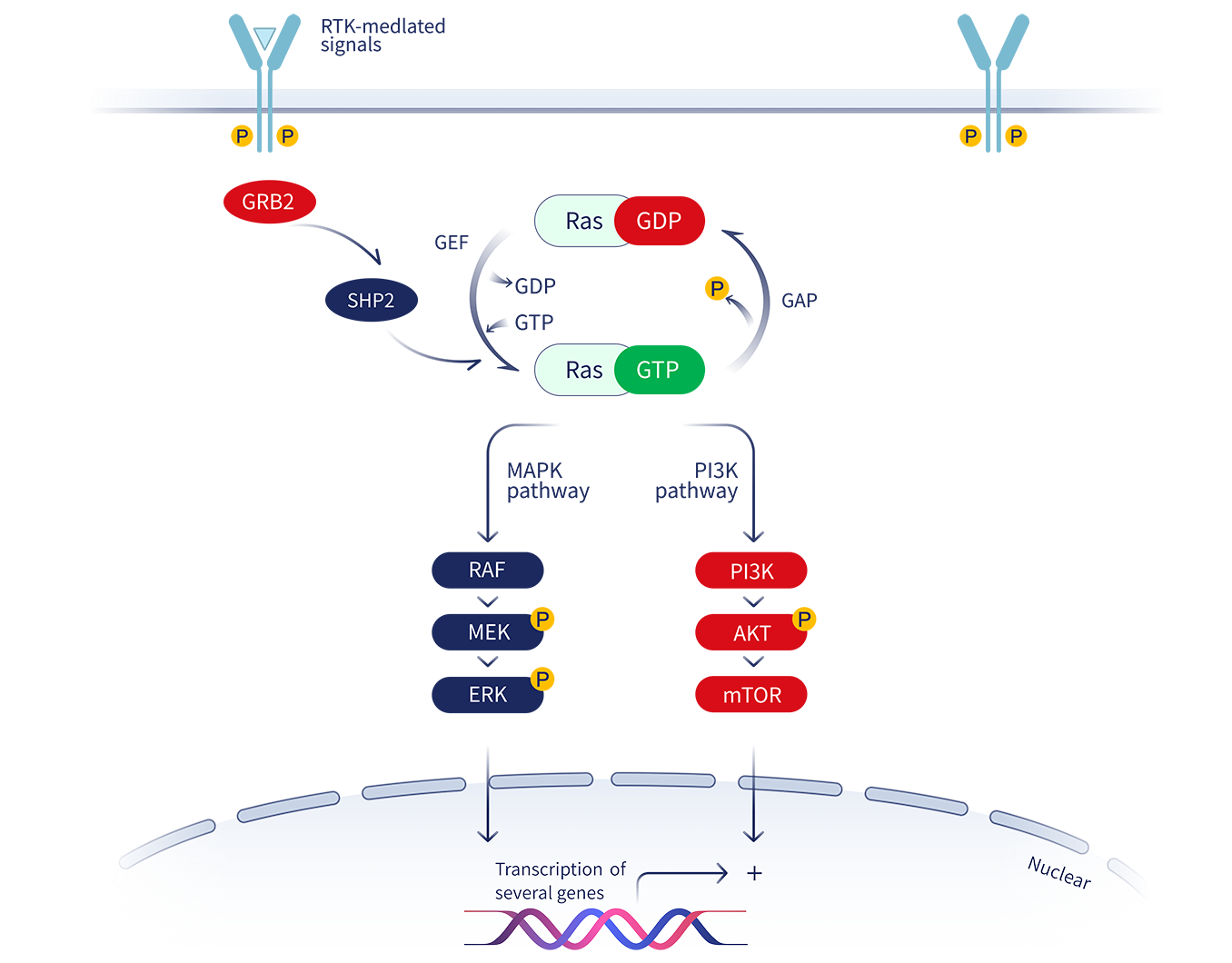
Kirsten rat sarcoma (KRAS) belongs to the family of small GTPases and is one of the most frequently mutated genes in tumors. Its biological function in promoting tumor growth has been extensively studied. The mutation of glycine to cysteine (G12C) at position 12 of the KRAS protein constitutively activates downstream signaling pathways such as RAF-MEK-ERK and drives tumor cells to proliferate continuously.
Although it was discovered nearly 40 years ago, KRAS has always been regarded as an “undruggable target” by the pharmaceutical industry due to the smooth surface of the KRAS protein and the lack of a small-molecule binding pocket. However, in 2013, Professor Kevan M. Shokat of the University of California, San Francisco discovered a new binding pocket, switch-II (S-IIP) that can be induced and brought a new dawn for the development of KRAS inhibitors.
Jacobio designed the small-molecule allosteric inhibitor Glecirasib using its own allosteric inhibitor platform. Glecirasib is a potent, irreversible KRAS G12C inhibitor. Glecirasib covalently binds to the mutated cysteine residue on site 12 of GDP-bound KRAS G12C. This locks KRAS G12C in an inactive state, blocking KRAS-dependent signal transduction. This inhibition results in favorable anti-tumor effects by reducing the growth and proliferation of tumor cells and inducing apoptosis.
Glecirasib has not only high selectivity and potency, but also has unique utility due to its unique molecular structure. The drug has demonstrated better efficacy and safety in early clinical studies compared other standard of care treatments. The drug also had an extremely low gastrointestinal toxicity profile that resulted in better overall patient compliance. Glecirasib is potentially a best-in-class drug and is expected to benefit non-small cell lung cancer and colorectal cancer patients with KRAS G12C mutations.
Jacobio Pharma presented clinical results of glecirasib at the JCA-AACR Precision Cancer Medicine International Conference

Jacobio presented Phase I clinical data of KRAS G12C inhibitor Glecirasib at the 2022 annual meeting of American Society of Clinical Oncology (ASCO)

Jacobio presented the results of Glecirasib as a single agent or in combination with JAB-3312 in preclinical cancer models during the 2022 European Society of Medical Oncology ASIA (ESMO ASIA)
